- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
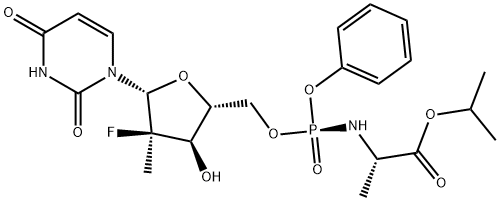
Sofosbuvir
Product Name: Sofosbuvir
Formulae hypotheticae: C22H29FN3O9P
M. Pondus; 529.45
CAS Subcriptio Number: 1190307-88-0
Model:CAS NO:1190307-88-0
Mitte Inquisitionem
Sofosbuvir
Product nomen:Sofosbuvir 1190307-88-0CAS No:1190307-88-0
Specification: in domo
Nomen
Sofosbuvir
Synonyma
(S)-isopropyl2-(((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyriMidin-1(2H)-yl)-4-fluoro -3-hydroxy-4-Methyltetrahydrofuran-2-yl)Methoxy)(phenoxy )phosphoryl)aMino)propanoate;PSI-7977 Discontinued;L-Alanine,N-[[P(S),2'R]-2'-deoxy-2'-fluoro-2'-Methyl-P-phenyl-5' -uridylyl]-,1-MethylethylChemic albookester;SophyBouvet;Sofosbuvirandintermedia;Sofosbuvir1190307-88-0; Isopropyl(2S)-2-{[(S)-{[(2R,3R,4R,5R)-5-(2,4-dioxo-3,4- dihydro-1(2H)-py rimidinyl) -4-fluoro-3-hydroxy-4-methyltetrahydro-2-furanyl]methoxy}(phenoxy)phosphoryl]amino}propanoate;PSI-7977;GS-7977;PSI7977;GS7977;SOFOSBUVIR

M
![CAS # 59-02-9, Vitamin E, D-alpha-Tocopherol, (2R)-3,4-Dihydro-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-2H-1-benzopyran-6-ol](https://i.trade-cloud.com.cn/upload/6698/image/20230529/1190307-88-0_255833.gif)
Formulae hypotheticae
C22H29FN3O9P
M. Pondus
529.45
CAS Subcriptio Number
1190307-88-0
Pertractatio et repono
Cautiones pro tutum pertractatio
Bene ventilatas tractantem in loco. Apta tutela vestitus. Pelle et oculis contactum fuge. Fuge formationem pulveris et aerosols. Utere instrumentis non-noctis. Ne ignis per electrostatic missionem vaporis.
Conditiones repono pro tuto, inter quaslibet incompatibilitates
Repone continens arcte clausum in loco sicco, frigido et bene ventilato. Disponunt a vasis victualibus vel materiae repugnantibus.
Imprimis usus finis (s): oeconomiae laboratoriae, ad investigationes scientificas et evolutionem tantum